War on Bugs: Will Antibiotic Resistance Usher in a 'Medical Dark Age'?
Late last year a woman in her 70s died in a Reno hospital in the state of Nevada, in the United States. This, while sad, would usually be totally unremarkable were it not that the cause of death was infection by a bacteria resistant to every antibiotic available in the US.
This isn’t the first time that somebody has died from infection by an antibiotic-resistant ‘super bug’. In fact, it is estimated that in the US alone, over 2 million people are infected with antibiotic-resistant bacteria every year, and at least 23,000 people die from these infections. Although antibiotic resistance is now considered a significant threat to public health, the issue isn’t a new one.
Alexander Fleming, the scientist who discovered penicillin, cautioned against the overuse of the ‘miracle drug’ after it became available for mass production and use in 1945. In an interview shortly after accepting a Nobel prize for his discovery, Fleming warned, “The thoughtless person playing with penicillin treatment is morally responsible for the death of the man who succumbs to infection with the penicillin-resistant organism.”
Only three years later, in 1948, Harvard Magazine reported a four-fold increase in resistant staph infections in hospitals. In 1952, the first scientific article on antibiotic resistance was published in the British Journal of Pharmacology. Despite these early warnings, antibiotic use was rife during the 1950s, 60s and 70s. By the 90s, public health campaigns started to emerge encouraging the cautious use of antibiotics.
More recently, an investigation commissioned by UK Prime Minister David Cameron in 2014 estimated that in the near-future antibiotic resistance would be the cause of 10 million deaths per year, and a $100 trillion cost to the UK’s gross national product. Late in 2016, the United Nations General Assembly took up the issue of antibiotic resistance, making antibiotic resistance along with HIV, noncommunicable diseases, and Ebola, one of only four issues ever addressed by this group.
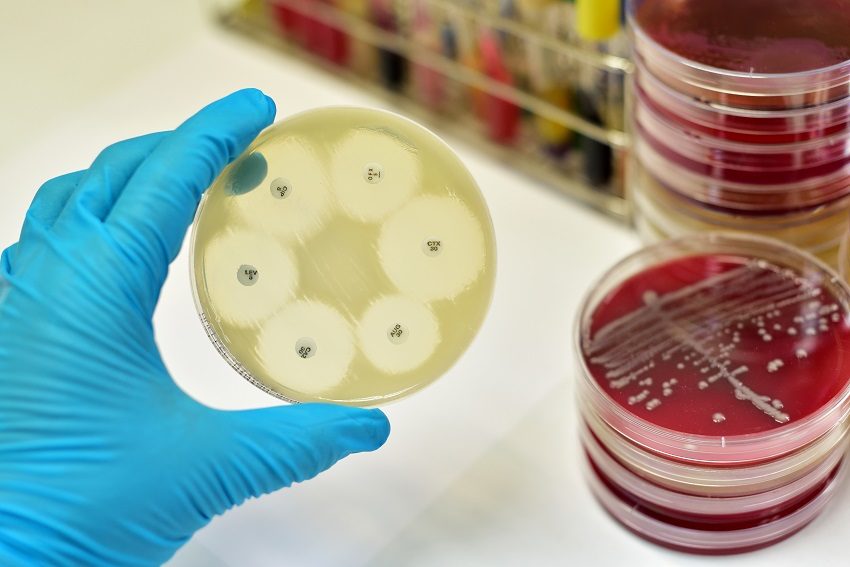
So how, amidst warnings delivered hand-in-hand with the very first antibiotic, did we get into this pickle? Primarily, overuse. A 2012 study published in the Internal Medicine Journal found that in one Australian hospital, 47 per cent of antibiotic use was contrary to guidelines and/or microbiological results. The problem with this is not just that the patient may not get the best immediate outcome, but that every time an antibiotic is used, resistance grows. The mechanism? Only the most well-known principle of evolution: natural selection.
 Over-use of antibiotics is resulting in the emergence of bacteria resistant to the ‘miracle’ drug
Over-use of antibiotics is resulting in the emergence of bacteria resistant to the ‘miracle’ drug
Certain bacteria are susceptible to certain antibiotics. For example, if you have strep throat, you need penicillin; if you have chlamydia, you need tetracycline (and perhaps a stern talking to about sexual health). When an antibiotic is used it puts selective pressure on bacteria by killing the ‘susceptible’ bacteria causing the infection, but allowing the survival of the bacteria that are naturally resistant to the antibiotic.
As a result, the remaining antibiotic-resistant bacteria have an advantage, and pass this advantage on to their offspring via vertical gene transmission. Thus, a new and improved generation of antibiotic-resistant bacteria. Those cunning superbugs can also pass on the resistance gene to bacteria of other species via horizontal gene transmission. Horizontal gene transmission is when bacteria ‘share’ genetic material with other bacteria. How nice of them.
Even more hideous than this army of mutant bacteria genetically outsmarting modern medicine is that people also spread these antibiotic-resistant bacteria to other people, via kissing, coughing, touching unwashed hands, and other gross activities. They can also spread via airplane, water, and in the wind. If livestock are given antibiotics to promote growth (a practice that is now largely prohibited) or to treat illness, then they too can develop resistance. These bacteria can spread to humans via traces remaining on meat, or via the consumption of crops that have been treated with fertiliser or water containing traces of animal faeces.
Throughout the relatively brief history of antibiotic discovery and use, an increase is antibiotic resistance has always followed the introduction of new drug classes. Given there has not been a new class of antibiotics discovered since the 1980s, it seems that we may have been officially out-smarted. And if we have then we could be headed for a medical dark age, one in which the WHO has warned “many common infections will no longer have a cure and, once again, could kill unabated”.
Dr Jessica L Paterson, Senior Research Fellow, CQUniversity, Appleton Institute